ALUMINUM
SULFATE
PRODUCT IDENTIFICATION
10043-01-3
TOXICITY
SMILES
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white lump or powder
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
http://www.dnr.state.wi.us/
What
is alum and how doesit work?: ALUM (aluminum sulfate) is a nontoxic
material commonly used in water treatment plants to clarify drinking
water. In lakes alum is used to reduce the amount of the nutrient
phosphorus in the water. Reducing phosphorus concentrations in lake
water can have a similar clarifying effect by limiting the availability
of this nutrient for algae production. Phosphorus enters the water
either externally, from run-off or ground water, or internally,
from the nutrient rich sediments on the bottom of the lake. Phosphorus
is released from the sediments under anoxic conditions that occur
when the lake stratifies and oxygen is depleted from the lower layer.
Even when external sources of phosphorus have been curtailed by
best management practices, the internal recycling of phosphorus
can continue to support explosive algal growth. Alum is used primarily
to control this internal recycling of phosphorus from the sediments
of the lake bottom. On contact with water, alum forms a fluffy aluminum
hydroxide precipitate called floc. Aluminum hydroxide (the principle
ingredient in common antacids such as Maalox) binds with phosphorus
to form an aluminum phosphate compound. This compound is insoluble
in water under most conditions so the phosphorus in it can no longer
be used as food by algae organisms. As the floc slowly settles,
some phosphorus is removed from the water. The floc also tends to
collect suspended particles in the water and carry them down to
the bottom, leaving the lake noticeably clearer. On the bottom of
the lake the floc forms a layer that acts as a phosphorus barrier
by combining with phosphorus as it is released from the sediments.
Local: APPLICATION: Coagulant in pulp and paper mills, water purification and treatment, leather tanning, textiles; lubricating compositions,fire retardants; decolourisaton agent in petroleum , deodorizer; food additive; firming agent; sizing paper; lakes; dyeing mordant; foaming agent in firefighting foams; fireproofing cloth; catalyst, pH control ; waterproofing concrete
GENERAL DESCRIPTION OF ALUMINUM: Aluminum (Aluminium in British English) is a silver-white ( with a face-centered cubic crystalline structure), ductile and light metal element in the member of group IIIa of the periodic table. Symbol Al; Atomic number 13; atomic mass 26.98154; melting point ca 660°C; boiling point ca 2,467°C; specific gravity 2.6989 at 20°C; valence +3; electronic config. [Ne]3s23p1. Aluminium crystallizes in a face-centered cubic lattice that is stable from 4 K to melting point. It is an excellent conductor of heat and electricity (60% of copper's). The coordination number is 12, it is light, malleable soft. Though pure aluminium is soft and lacks strength, it imparts a variety of useful properties including strong hardness when alloyed with small amounts of Cu, Mg, Si, Mn and other elements. Aluminum is very reactive chemically but it resists corrosion by the self-protecting continuous thin layer of oxidation which forms quickly on the nascent aluminium surface when exposed to oxygen, water or other oxidants and prevents further corrosion. The chemical properties of aluminium resemble those of beryllium and silicon. Due to its amphoteric character, it is rapidly attacked by alkalis (such as lye) and by mineral acids. Aluminium begins to polymerize when the pH of an acidic solution increases notably over pH 4.5. Polymerization implies two hydroxyls shared by two aluminium atoms in the first step, e.g., 2 Al(OH)(H2O)52+ -> Al2(OH)2(H2O)84+ + 2 H2O. Polymerization gradually proceeds to larger structures, eventually leading to the formation of the Al13 (polycation). As polymers coalesce, they increase in relative molecular mass, eventually becoming large enough to precipitate aluminium hydroxide from solution. Aluminum is one of the most abundant metals in the earth. It is not found in nature as the free element but in combination in clay, bauxite, mica, feldspar, alum, cryolite, and in the several forms of alumina such as emery, corundum, sapphire, and ruby (forms of aluminum oxides). Aluminum is very important in world economy. Aluminium is allied to form many hard, durable, light, corrosion-resistant and readily worked into a variety of shapes which are vital to the building, transportation, aerospace and consumer durable goods industries. The development of aluminum coating that reflects both visible light and radiant heat is useful in the industry of telescope mirrors, jewelry and colored wall covering. Aluminum powder is used in paints and in welding with iron oxide. The mixture (called thermite) gives off large amounts of heat when ignited. Finely divided aluminium dust can ignite and cause explosions. It is used in making explosives. Aluminum is used in packing industry as cans and foil. Owing to the high ratio of Al3+ in aqueous solutions, the ion proteolyses part of the water envelope and forms hydroxo complexes. It can also complex with electron-rich species, such as fluoride and chloride. Commercial aluminum compounds in chemical industry are:- Alum: Various isomorphous solid sulfates composed of trivalent metals and univalent metals, especially aluminum potassium sulfate, AlK(SO4)2·12H2O, a white, crystalline compound. Alums have the general formula M2SO4·MIII2(SO4)3·24H2O, where M is one of alkali metals (potassium, sodium, rubidium, caesium, silver. thallium or ammonium), and MIII denotes one of the trivalent cation (e.g., aluminum, chromium, iron, manganese, cobalt, or titanium). In aqueous solution, alums show all the chemical properties that their components show separately. These salts are used in water purification, leather tanning, coagulation agent for rubber latex, mordant dyeing, fireproofing textiles, modifying concrete, baking powder, preparation of lakes, clarifying of turbid liquids and as astringents.
- Potassium aluminum sulfate (KAl(SO4)2·12H2O, CAS RN: 7784-24-9 (Dodecahydrate), 10043-67-1 (Anhydrous))
- Sodium aluminum sulfate (NaAl(SO4)2·12H2O, CAS RN: 10102-71-3)
- Ammonium aluminum sulfate (NH4Al(SO4)2·12H2O, CASR RN: 7784-25-0 (Anhydrous), 7784-26-1 (Dodecahydrate))
- Chromium potassium sulfate (KCr(SO4)2·12H2O, CAS RN: 10141-00-1 (Anhydrous), 7788-99-0 (Dodecahydrate))
- Aluminum fluorosulfate (FAl(SO4)2·12H2O, CAS RN: 73680-58-7)
- Alumina (Al2O3, CAS RN: 1344-28-1, 11092-32-3): White crystalline powder that is found as balls or lump of various mesh sizes. Aluminium oxide is found in different modifications. The natural form occurs as corundum (alpha-Al2O3) or in hydrated forms. The hexagonally closest-packed alpha-modification (corundum) is the most stable oxide. Emery is an abrasive composed of pulverized, impure corundum, and ruby and sapphire are the gem varieties of the mineral corundum occurs as masses in limestone and as segregations in igneous rock. All transitional aluminas produced at low temperatures converts to alpha-alumina at high temperature (1400°C) since a series of alumina formation by dehydration of the hydroxides contain a small proportion of hydroxyl groups and retaining some chemical reactivity. Example are gamma-aluminas (or activated aluminas) formed by dehydration at below 600°C and rho-aluminas formed by dehydration at higher temperatures (900-1000°C) which are nearly anhydrous Al2O3. The structural and compositional differences among various forms of alumina are associated with differing particulate size, particulate surface area, surface reactivity and catalytic activity. Alumina is used in abrasive and as a adsorbent as well as in manufacturing other aluminum compounds, paper, spark plugs (Alumina porcelain), fluxes, heat resistance fiber and chromatographic analysis. The form of balls to inch (6.4 to 19 millimeters) in diameter are used in reactor and catalytic beds. Alumina bricks containing 50, 60, or 70% alumina are used in high temperature applications. Alumina bubble bricks which are manufactured by passing an air jet over molten alumina to produce small hollow bubbles are used to line kiln walls. Alumina fibers (also known as sapphire whiskers), linear crystals of alumina which have a strength of up to 200,000 lb/in2 are used in plastics as a filler to improve heat resistance and dielectric properties. aluminous cements containing high percentage of alumina sets to a high strength in 24 hours and are used for constructing bank walls and laying roads. Various forms of aluminum oxides;
- Bayerite (CAS RN: 20257-20-9, alpha-aluminium trihydroxide, alpha-Al(OH)3 or alpha-Al2O33H2O)
- Boehmite (CAS RN:1318-23-6, gamma-AlO(OH) or gamma-Al2O3H2O)
- Corundum (CAS RN:1302-74-5, alpha-Alumina , Al2O3)
- Diaspore (CAS RN:14457-84-2, alpha-AlO(OH) or alpha-Al2O3H2O)
- Gibbsite (CAS RN: 14762-49-3, , gamma-aluminium, gamma-Al(OH)3 or gamma-Al2O33H2O)
- Nordstrandite (CAS RN: 13840-05-6, beta-aluminium trihydroxide, beta-Al(OH)3 or beta-Al2O33H2O)
- Aluminate: A negative ion usually assigned the formula AlO2- and derived from aluminum hydroxide.
- Aluminide: An intermetallic alloy containing aluminum plus another element, such as nickel, iron, or titanium.
- Aluminite (Al2(SO4)(OH)4·7H2O, also known as websterite) Native monoclinic hydrous aluminum sulfate; used in tanning, papermaking, and water purification.
- Aluminize :To apply a film of aluminum to a material, such as glass. To form a protective surface alloy on a metal by treatment at elevated temperature with aluminum or an aluminum compound.
- Aluminized explosive: An explosive to which aluminum has been added.
- Aluminized Steel: A steel coated with an aluminum-iron alloy coating; prepared by dip-coating and diffusing aluminum into steel at 870°C; resists scaling and oxidation up to 900°C. Also known as alumetized steel; calorized steel.
- Aluminum Alkoxides: used in varnishes, for textile impregnation, in cosmetics and as an intermediate in pharmaceutical production
- Aluminum Antimonide (AlSb); employed in the semiconductor technology industry
- Aluminum Borate: used in the production of glass and ceramics
- Aluminum Butylate (Al(OC4H9)3, CAS RN: 2269-22-9)
- Aluminum Chloride (AlCl3, CAS RN: 7446-70-0): Used as a catalyst in the process of Friedel Crafts. (It has an electron deficient molecule forming only 3 bonds, and has no lone pairs. The catalyst acts as an electron acceptor for a lone pair on the halide atom). It is widely used in the manufacturing of petrochemicals such as alkylbenzene, ethylbenzene, alkyl aryl ketone, ethyl chloride. It is also used in the manufacturing pharmaceuticals, dyes intermediates and other organics chemicals such as anthraquinone, phthalocyanines, acetophenone, butyl rubber, phenylethyl alcohols. It is used as a nucleu inhibitor in the production of titanium dioxide. Aluminum Chloride is also used in the production of aluminum, in the metallurgical industry and as a flux in aluminum smelting; in the production of rubber; lubricants and wood preservatives, and in cosmetics as an astringent; active ingredient in antiperspirants.
- Aluminum Chlorohydrate (AlCl(OH)5, CAS RN: 1327-41-9, 11097-68-0, 84861-98-3)
- Aluminum Fluoride (AlF3, 7784-18-1): used in aluminum production and ceramics and glass manufacturing, as a catalyst for organic synthesis, inhibitor of fermentation.
- Aluminum Halogenides, hydrides and lower aluminium alkyls react violently with molecular oxygen, and are spontaneously inflammable in air and explosive with water. Industrially these compounds are used as co-catalysts for organometallic and organic synthesis, and as intermediates in various production processes.
- Aluminum Hydroxide (Al2O3·3H2O, or Al(OH)3,CAS RN: 21645-51-2 ) Hydrated alumina, or simply hydrate, is more accurately chemically designated as aluminum trihydroxide, Al(OH)3. The aluminium hydroxides found abundantly in nature are gibbsite, diaspore, and boehmite. They all convert to aluminium oxide when heated. Aluminum Hydroxide is a non-abrasive powder with a Mohs' hardness index of 2.5 - 3.5 and a specific gravity of 2.42. Alumina trihydrate is the largest volume flame retardant used in the world. On heating to 200°C, hydrated alumina decomposes into 66% alumina and 34% water. This irreversible process is, in part, what makes ATH an effective flame retardant. Aluminium hydroxide is also used as an adsorbent, emulsifier, ion exchanger, mordant, antacid, and filtering medium. It is also used in the manufacture of paper, ceramics, printing inks, detergents, for waterproofing fabrics and in dentrifrices and antiperspirants
- Aluminum Isopropylate (Al(OCH(CH3)2)3, CAS RN: 555-31-7): used in the soap and paint industries; waterproofing textiles
- Aluminum Lactate (Al(C3H5O3)3, CAS RN: 18917-91-4)
- Aluminum Magnesium Silicate (MgAl2(SiO4)2, )
- Aluminum Nitrate (Al(NO3)3, CAS RN: 13473-90-0)
- Aluminum Orthophosphate (AlPO4, CAS RN: 7784-30-7): Flux for the production of glass, mixture of ceramic, waterproofing concrete and dental cements, in cosmetics as a emollient, flame retardant, catalyst in organic synthesis, fireproofing textile, pharmaceuticals, dyes.
- Aluminum Phosphide (AlP): used as a rodenticide and pesticide.
- Aluminum Selenide (AlSe): employed in the semiconductor technology industry
- Aluminum Silicates (Clay); They have cation-exchange capacity and the amounts and types of clay minerals in a soil largely determine its physical properties and suitability for agriculture. Used in component of dental cement; antacid, food additives
- Aluminum Sulfate: can exist with varying proportions of water, the common form being Al2(SO4)3•18H2O. It is almost insoluble in anhydrous alcohol, but readily soluble in water. Above 770°C decomposition to aluminium oxide is observed. Aluminium sulfate is mainly used in water treatment, dyeing, leather tanning and in the production of other aluminium compounds. Aluminum Sulfate (Alum) is a white crystalline product which is almost insoluble in anhydrous alcohol, but readily soluble in water. It decomposes to aluminium oxide on heating or on burning (above 770°C) producing toxic and corrosive fumes including sulfur oxides. Its can exist with a variable number of water molecules (n close to 18), the form being Al2(SO4)3•nH2O. It dissolves with clear water at the concentration of 1 - 5%. The solution in water is a medium strong acid reacts with alkalis and attacks many metals in presence of water. It is widely applied as a coagulant for clarification of water treatment for industrial and drinking and in dyeing, leather tanning, in paper production, as a mordant in dyeing, and as a starting material for the production of other aluminium compounds. Another application for hydrated alumina is for the manufacture of zeolites.
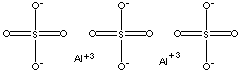
- aluminum Trisulfate (Al2(SO4)3, CAS RN: 10043-01-3)
- Ammonium Alum (NH4Al(SO4)212H2O, CAS RN: 7784-26-1)
- Anorthosite (Na2OAl2O36SiO2): Sodium calcium silicoaluminate
- Bauxite (CAS RN: 1318-16-7)
- Cryolite (Na3AlF6, CAS RN: 15096-52-3) Sodium calcium
- Kaolinite (Aluminium silicate, hydrate -Al2Si2O5(OH)4 )
- Potash Alum (K(AlO)3(SO4)212H2O, CAS RN: 7784-24-9)
- Sodium Alum (NaAl(SO4)212H2O, CAS RN: 7784-28-3)
- Sodium Aluminate (NaAlO2, Na2OAl2O3 or Na2Al2O4,, CAS RN: 1302-42-7)
- Sodium Aluminium Phosphate: used in food additives
- Topaz (Aluminium Silicofluoride, 2Al2O32Al(F,OH)33SiO2 )
- Trimethylaluminium (Al(CH3)3, CAS RN: 75-24-1)
FERRIC
APPEARANCE
white to off-white flake
ALUMINIUM (as Al2O3)
17.0 ~ 18.0%
IRON COMPOUND
0.5% max
INSOLUBLES IN WATER
0.3% max
pH
2.7 ~ 3.7 (5% Sol.)
HEAVY METAL (as Pb)
10ppm max
ARSENIC (as As)
3ppm max
NON-FERRIC
APPEARANCE
white to off-white flake
ALUMINIUM (as Al2O3)
17.0 ~ 18.0%
IRON COMPOUND
0.03% max
INSOLUBLES IN WATER
0.3% max
pH
2.8 ~ 3.8 (5% Sol.)
HEAVY METAL (as Pb)
10ppm max
ARSENIC (as As)
3ppm max
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H335 May cause respiratory irritation
H411 Toxic
to aquatic life with long lasting effects
H315 Causes skin
irritation
H319 Causes serious eye irritation
PRECAUTIONARY STATEMENTS
P261 Avoid breathing dust/fume/gas/mist/vapors/spray
P273 Avoid
release to the environment
P302+ P352 IF ON SKIN: Wash with
plenty of soap and water
P280 Wear protective gloves/protective
clothing/eye protection/face protection
P305 + P351 + P338 IF
IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing
![]()
![]()
RISK
36/37/38 Irritating to eyes, respiratory system and skin.
51/53 Toxic
to aquatic organisms, may cause long-term adverse effects in the
aquatic environment.
SAFETY
26 In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice.
29 Do not empty
into drains
37/39 Wear suitable gloves and eye/face protection.
61
Avoid release to the environment. Refer to special instructions
/ safety data sheets.
PRICE INFORMATION